Background, interpretation, and significance of our paper on Biasing reaction pathways with mechanical force
Electrocyclic ring closing reactions are molecular rearrangements in which a cyclic structure is formed from a linear π system. Specifically, a new single bond is generated between the termini of the π system, and the product has two fewer π electrons. The converse process is known as electrocyclic ring opening.
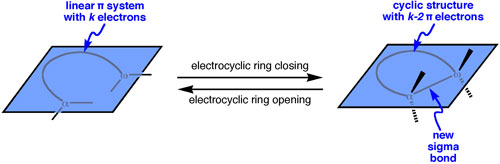
Electrocyclic reactions are generally thought to occur by a concerted mechanism. In concerted mechanisms, bonds are made and broken simultaneously, by a process that involves no intermediates. The orbitals responsible for chemical bonding in the reactants must transform smoothly into those used for bonding in the cyclic product. What is meant by “transform smoothly”? It means that there is congruence between the orbital symmetry characteristics of the reactants and products. This principle is known as the conservation of orbital symmetry; Woodward and Hoffmann described the details of this principle in 1969 (Angew. Chem. Int. Ed. 1969, 8, 781-853).
For concerted mechanisms, the motion traced out by the atoms in the course of a reaction – the reaction pathway – is governed by the principle of orbital symmetry. The electrocyclic process can either go by way of motion that is called conrotatory or disrotatory. Conrotatory motion means that the terminal atoms twist in the same direction (both clockwise or both counterclockwise). In contrast, disrotatory motion means that the two termini twist in opposite directions.

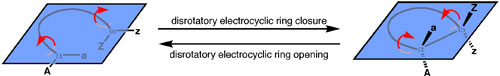
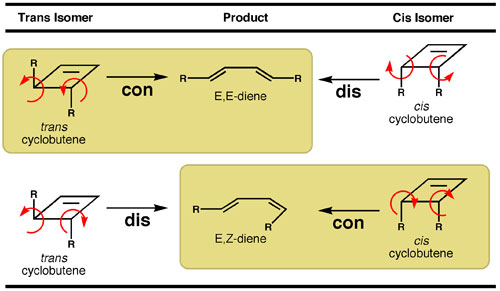
The conservation of orbital symmetry principle makes predictions about the pathway with which electrocyclic reactions will follow. For a linear system having four-π electrons, orbital symmetry is conserved when the conrotatory pathway is followed. In contrast, the disrotatory pathway for a four-π electron system experiences a very large, symmetry-imposed barrier. These considerations in turn make predictions about the products that will be obtained in electrocyclic ring opening reactions (Table 1). Starting from a trans cyclobutene, electrocyclic ring opening via conrotatory motion will produce an E,E-diene. In contrast, starting from the cis cyclobutene isomer, conrotatory motion will yield an E,Z-diene. The predictions made by orbital symmetry have generally been found to hold true in experimental observations.
Table 1. Conservation of orbital symmetry predicts that cyclobutenes undergo electrocyclic ring opening by the conrotatory pathway (shaded boxes). The trans and cis isomers result in diene products that have different double bond geometry.
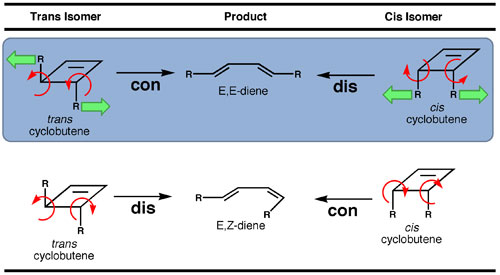
We wondered if mechanical force – specifically a tensile or stretching force – applied to the substituents of a cyclobutene would influence the observed electrocyclic reaction pathways. We surmised that depending on the cyclobutene geometry, force could either act to increase the reaction rate, slow down the rate of the reaction, or possibly even override the pathways preferred by orbital symmetry. The intuitive reason why force might play a decisive role can be seen from Table 2. As the substituents of the trans isomer are pulled apart, the atoms twist by a motion that closely follows the allowed, conrotatory pathway and leads to the E,E-diene. However, pulling apart the substituents on the cis isomer is expected to twist the atoms in a way that resembles the disrotatory pathway. If the cis isomer were to undergo disrotatory ring opening, it too would result in the E,E-diene rather than the E,Z-diene that is expected from the concerted, orbital symmetry allowed conrotatory pathway. To summarize, knowing the starting cyclobutene isomer and the geometry of the diene product, it is thus possible to tell if the reaction follows the disrotatory or conrotatory pathway.
Table 2. The intuitive action of tensile force acting on the cyclobutene substituents (represented by the green arrows) is expected to favor the conrotatory pathway for the trans isomer, and the disrotatory pathway for the cis isomer. The consequence is that both isomers will result in the same product (shaded box).
Finding a way to introduce the tensile force was a key finding. It had been known for sometime that polymers exposed to ultrasound underwent covalent bond scission in the middle of the chain due to shear-induced, tensile-like forces. What had never been done previously was to use such forces to accelerate or change the course of a rearrangement reaction. Consistent with the expectations in Table 2, our experiments showed that both the cis and trans isomers of benzocyclobutene both gave the E,E-product, indicating that the two isomers followed con and disrotatory pathways, respectively. This finding provided strong evidence to suggest the role of force in biasing reaction pathways.
Questions remain about the mechanism. For the cis isomer, the force-induced disrotatory pathway is forbidden by symmetry, assuming that the reaction remains concerted. At least two possibilities can be considered to explain how force could produce the observed result. Either the mechanical energy associated with the force has been sufficient to overcome the symmetry forbidden process, or force has opened a new pathway that does not follow a concerted mechanism. From the experiments we have performed thus far, it is not possible to conclude which of these explanations, if either, account for the observations.
Reference:
Charles R. Hickenboth, Jeffrey S. Moore, Scott R. White, Nancy R. Sottos, Jerome Baudry, Scott R. Wilson, ''Biasing reaction pathways with mechanical force'', Nature, 447, 2007, pp. 423-427.



